Pella Corporation Waste Cost Reduction
June 21st, 2014 Posted by admin Home No Comment yet
Pella Corporation, a window and door manufacturer, reduced releases and transfers of 33/50 Program chemicals from 896,300 pounds in 1988 to 151,750 pounds in 1994, an 83 percent decrease. These reductions were achieved primarily by eliminating solvents from adhesives, paints, and cleaning processes. This Success Story focuses on an innovative method to reduce and potentially eliminate chromium releases by using carbon adsorption to recover chromium from industrial wastewater.
Pollution Prevention Awards for Environmental Excellence

PARENT COMPANY BACKGROUND
Pella Corporation, founded in 1925, is a privately held company that manufactures windows and doors (SIC 2431–Millwork). Known for years as the Rolscreen Company, the business grew on the strength of the residential housing market. In 1990, the company changed its name to Pella Corporation.
Pella markets several different lines of windows and doors. Pella Designer Series®, Pella Architect Series™, and Precision Fit® products are manufactured at company headquarters in Pella, Iowa, and sold through a nationwide network of Pella Window Stores®. In addition, Pella ProLine® products are manufactured in Carroll, Iowa, and sold through nationally
recognized building supply retail outlets. Pella also serves architectural firms by designing and manufacturing custom windows and doors at its plant in Shenandoah, Iowa. Pella employs approximately 3,500 people.
COMPANY 33/50 PROGRAM GOALS & ACHIEVEMENTS
Pella’s management initiated the efforts to join the 33/50 Program in 1991 and established a goal to significantly exceed EPA’s 33/50 targets by reducing 33/50 chemical releases and transfers by 90 percent by 1995, using 1988 as the baseline year. The company reduced 33/50 chemical releases and transfers by 65 percent in 1992 and by 83 percent in 1994. This was accomplished by switching to non-solvent-based adhesives; selecting high-solids paints; eliminating perchloroethylene emissions by replacing one vapor degreaser with an aqueous parts washing system and eliminating the need for a second vapor degreaser by changing the materials used in window manufacturing; and eliminating methylene chloride emissions by simplifying a glazing production process.
FEATURED POLLUTION REDUCTION PROJECT:
ACTIVATED CARBON FOR CHROMIUM RECOVERY FROM INDUSTRIAL WASTEWATER
Pella paints aluminum extrusions in order to give customers a product with a superior maintenance-free
exterior. Aluminum extrusions are processed through a five-stage chromium conversion process prior to adding a durable paint finish. Wastewater from this process contains hexavalent and trivalent chromium. In the past, the wastewater was treated by an in-house wastewater plant using a standard chromium reduction/chemical precipitation process. Consequently, a chrome sludge was produced and disposed of in accordance with the requirements of the Resource Conservation and Recovery Act. Effluent wastewater was regulated under a pretreatment agreement with the local POTW. The total annual cost of operating the wastewater treatment system was approximately $187,000.
Since chrome sludge was the company’s largest hazardous waste stream, staff engineers searched for ways to eliminate chromium from the production process. They investigated chromium-free pretreatment methods, but these alternatives did not pass internal product quality standards for paint durability. In 1994, Pella
learned about a metals recovery and recycling process developed by Lewis Environmental Services, Inc. (LES) of Pittsburgh, Pennsylvania. The recovery system utilizes granular activated carbon with a proprietary conditioning pretreatment to enhance heavy metal adsorption, combined with electrolytic metal recovery to produce a valuable metallic product. LES has a patent pending for this system, which is called the Enviro- Clean process. Pella requested LES to demonstrate the applicability of the process to chromium. Additional sampling was done to characterize the wastewater and a treatability study was conducted. It became apparent that a properly designed system would meet the following goals: (1) reduce or eliminate chrome sludge; (2) recycle wastewater back into conversion coating rinses; (3) achieve cost savings by reducing chemical purchases and hazardous waste disposal.
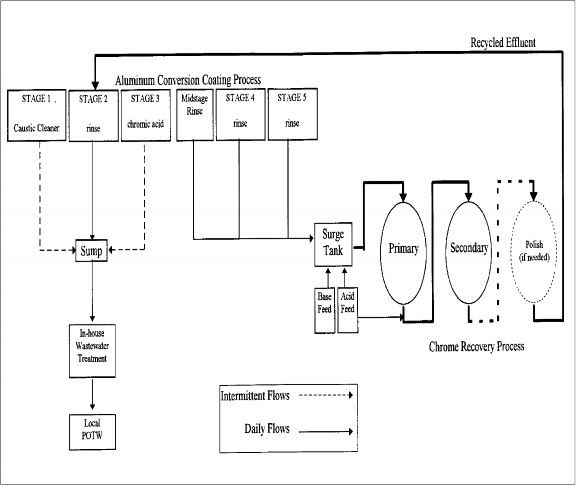
LES designed and built a system consisting of two adsorbers operating in series. A schematic of the system is shown in Exhibit 1. Influent wastewater flows into a 500-gallon surge tank equipped with chemical feed mechanisms used to adjust the pH to approximately 4.5. Wastewater is pumped through the primary adsorber, where more than 90 percent of the chromium is removed. Since the pH tends to rise slightly as the wastewater flows through the primary adsorber, a midstage pH meter and chemical feed maintains an optimum pH as wastewater moves into the secondary adsorber. Operators monitor the chromium concentrations of the influent, midstage, and the effluent twice per 10-hour shift to determine system effectiveness and performance. Once breakthrough occurs in the primary adsorber, it is sent offsite for regeneration and replaced by the secondary adsorber, which in turn is replaced by a recently regenerated adsorber.
The modular design of the system allows for operational flexibility in meeting the wastewater treatment requirements. Quick-disconnect hoses allow for rapid changes of the adsorbers. In addition, it is possible to run additional adsorbers in a series of two or three units, thereby increasing the capacity of the system. Consequently, Pella purchased a system with five adsorbers and the capacity to run 40 gallons per minute (gpm). The total cost of purchasing, installing, and modifying the system in two phases was approximately
$115,000.
The first phase of the project, which began in November 1995, involved equipment installation and startup. Initially, wastewater from all five stages of the aluminum conversion coating process was treated with two adsorbers operated in series. However, because the rate of aluminum carryover was higher than expected, an aluminum hydroxide precipitate formed in the surge tank and subsequently accumulated in a layer that restricted flow to 8 gpm in the first adsorber. Therefore, plumbing modifications were made so that in the first project phase, only rinses from the fourth and fifth stages were sent to carbon adsorbers while wastewater from the other three stages was treated by conventional chromium reduction/precipitation. The rinsewater flows from the fourth and fifth stages had chromium concentrations ranging from 60-80 mg/L and flowed at 15 gpm for one 10-hour shift per day. In addition, the influent pH was lowered to 4.0 in order to minimize aluminum hydroxide precipitation, maintain normal flow rates, and still achieve a chromium removal rate of 99 percent. Under this configuration, the system removed more than 99 percent of the chromium. Chrome sludge disposal was reduced by 32 percent during the first phase of the project.
The second phase, which began in May 1996, involved plant modifications to incorporate additional wastewater flows from a second chromium conversion line and to capture the midstage rinse following the third stage. After completing the modifications, the influent concentration of chromium increased significantly to 200-500 mg/L, the flow rate increased to approximately 27 gpm, and the effluent was recycled back into the production process. Due to the higher chromium loadings, three adsorbers were run in series in order to achieve the desired chromium removal rates.
The advantages offered by the chromium recovery process include:
- Significantly reducing and potentially eliminating chrome sludge disposal. The recovered chromium material is sold to industrial users and completely spent carbon is sold as feedstock to steel manufacturers.
- Lowering operating costs. Implementing the carbon adsorption system generated an annual cost savings of approximately $77,000, due primarily to reduced purchases of the chemicals used in the traditional reduction/precipitation process. The company estimates that the payback period for the initial investment in the carbon adsorption system will be less than two years.
- Recycling effluent. The company consumes less water by recycling the effluent for use in a rinse stage earlier in the aluminum conversion process.
- Increasing operational flexibility through a modular design. The company can continue to adapt the system to accommodate changes in their production needs.
- Occupying less floor space than the traditional wastewater treatment system.
- Pella perceives the potential to adapt the carbon adsorption system in the future so that it can be used to treat all of the wastewater from the aluminum conversion process and virtually eliminate chrome sludge production. Due to the high concentration of dissolved aluminum in wastewater from the first two stages and the resulting precipitation of aluminum hydroxide in the surge tank, this adaptation presents some technical challenges that are currently being investigated by Pella.